Current evidence for intrahepatic cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver tumour, characterized by high mortality due to invasiveness, advanced stage at diagnosis, and resistance to systemic treatments [1,2]. Surgical resection, the primary curative treatment, is feasible for fewer than 20% of patients and is associated with high post-resection recurrence rates [1,2,4]. Liver failure, primarily caused by parenchymal loss, vascular or biliary obstruction, is the primary cause of death in patients with ICC [3]. To address such challenges, interdisciplinary management along with the use of systemic and locoregional therapies are routinely applied in clinical practice aiming to achieve effective local tumour control while preserving enough liver function.
Ablation for small ICC – What do we know?
Liver-direct locoregional therapies are increasingly investigated for intrahepatic cholangiocarcinoma, particularly in cases of liver-only disease, which may have a better prognosis compared to extrahepatic disease [5]. In this context, image-guided ablative therapies are emerging as an alternative therapy for small ICCs with contraindications to surgery and recurrent/residual tumours, showing promising results with similar median overall survival to repeated resection [6].
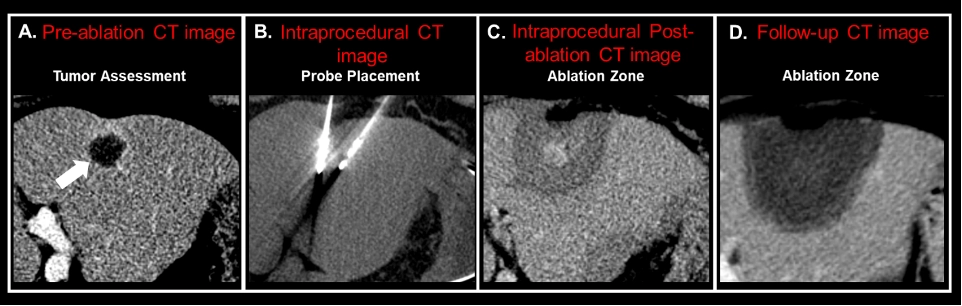
Percutaneous thermal ablation (PTA) provides several advantages due to its minimally invasive nature, enabling multiple retreatments, and favourable outcomes regarding complication rates, health-related costs, and recovery time. Additionally, PTA shows promise across various treatment scenarios for intrahepatic cholangiocarcinoma, including as a potential primary treatment, adjuvant therapy alongside surgery, for disease recurrence post-resection, and as a palliative option for unresectable disease [7] (Figure 1). A maximum diameter of 3 cm has been proposed as a threshold for both primary and recurrent intrahepatic cholangiocarcinoma due to its impact on complete ablation and local tumor progression rates, consistent with existing liver ablation literature for other tumor types [8,9]. This size cut-off also aids in distinguishing anatomo-pathological findings, such as a lower incidence of well-differentiated cholangiocarcinoma without microvascular invasion from poorly differentiated tumors with microvascular invasion [8].
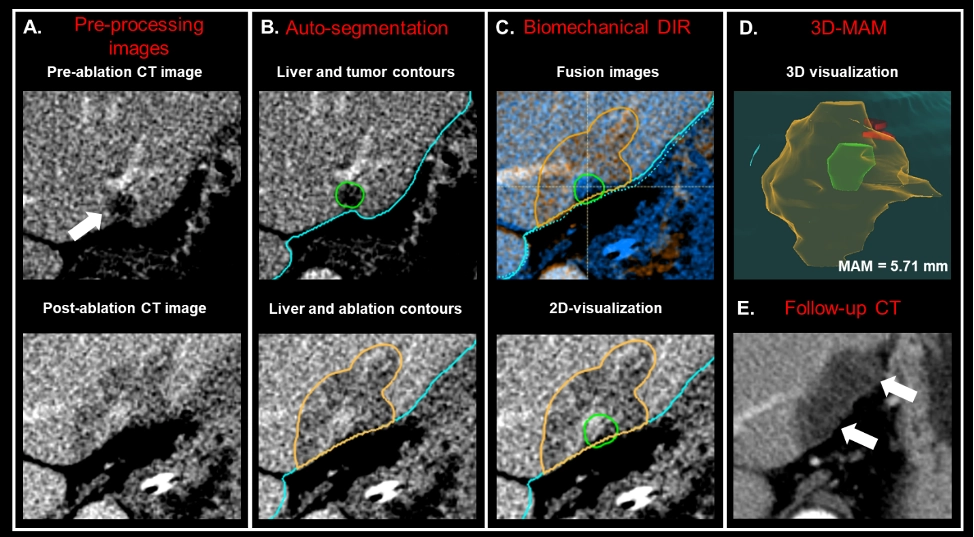
The prognosis of patients with ICC is influenced by factors such as tumor size, lymph node status, vascular invasion, pathology, and margin width [2]. Currently, the minimal ablative margin (MAM) associated with acceptable local tumor progression (LTP) rates for ICC is extrapolated from studies on hepatocellular carcinoma and colorectal cancer liver metastases (CLMs), typically falling within the range of 5-10 mm [10-14]. However, ICC may require larger ablative margins due to its infiltrative nature and high LTP rates [6]. In a retrospective analysis utilizing biomechanical deformable image registration (DIR) for intra-procedural ablative margin quantification (Figure 2), percutaneous thermal ablation (PTA) has demonstrated effectiveness as a local therapy for small ICCs, providing comparable survival outcomes to surgery. Despite these promising findings, further investigations with a larger patient sample and extended follow-up are required to confirm these results.
Future directions and conclusion
The increasing availability and recent technical refinements of percutaneous thermal ablation (PTA), along with standardization opportunities provided by stereotactic and robotic ablation methods, are poised to broaden indications and enhance the effectiveness of ablation as an alternative for patients with intrahepatic cholangiocarcinoma. Additionally, advancements in tumour molecular profiling, coupled with the development of systemic treatments targeting specific genetic alterations, along with AI-based prediction models, are expected to optimize patient selection and enable the precise use of ablation in ICC management.